Feline Coronavirus Antibody-Heartworm-Feline Leukemia Virus Antigen-Feline Immunodeficiency Virus Antibody Combo Test
คุณสมบัติสินค้า:
SKU : VD047
FIV Ab-FELV Ag-FCOV Ab-HW Ag
หมวดหมู่ : Rapid Diagnostic Test Kit , Feline Rapid Test , VET DIAGNOSTIX (China) ,
Share
Feline Coronavirus Heartworm Immunodeficiency Leukemia Virus Combo Test kit is a lateral flow immunochromatographic test for the qualitative detection of feline immunodeficiency virus antibody (FIV Ab), feline leukemia virus antigen (FeLV Ag), feline coronavirus antibody (FCoV Ab) and heart silk The worm antigen (CHW Ag) is found in serum, plasma and whole blood samples of cats.
Analysis time: 5-10 minutes
| Product Name | Feline Coronavirus Heartworm Immunodeficiency Leukemia Virus Combo Test kit |
| Specimen | serum, plasma and whole blood |
| Part No. | VD047 |
| Storage Temperature | 2-30°C, DO NOT FREEZE. Do not store the test kit in direct sunlight. |
PRINCIPLE
The Feline Coronavirus Heartworm Immunodeficiency Leukemia Virus Combo Test kit based on sandwich transverse flow immunochromatographic analysis. The test card has a test window for observing test runs and result readings. Before running a test, the test window has an invisible T (test) region and a C (control) region. As the treated sample enters each sample hole in the device, the fluid flows across the surface of each strip and reacts with the pre-coated FIV recombinant antigen, FeLV antibody, FCoV recombinant antigen, and cardifila antibody. If the specimen contains FIV antibody, FeLV antigen, FCoV antibody, and heartworm antigen, a visible T line will appear in the corresponding window. The c-line should always appear after application of the sample, which indicates that the results are valid. In this way, the device can accurately indicate the presence of FIV antibodies, FeLV antigens, FCoV antibodies, and heartworm antigens in the sample.
REAGENTS AND MATERIALS
-10 test pouches, with cards and disposable droppers
-10 vials of FIV-FeLV-CHW assay buffer (500μL each)
-10 vials of FCoV Ab assay buffer (1000μL each)
-1 package insert
SPECIMEN PREPARATION AND STORAGE
1. Specimen should be obtained and treated as below.
-Serum or plasma: collect the whole blood for the patient cat, centrifuge it to get the plasma, or place the whole blood into a tube which contains anticoagulants to get serum.
-Whole blood: collect fresh blood for use or make anticoagulant blood for storage at 2-8℃.
2. All specimen should be tested immediately. If not for testing right now, they should be stored at 2-8℃.
TEST PROCEDURE
-Allow all materials, including specimen and test device, recover to 15-25℃ before running the assay.
-Take out the test card from the foil pouch and place it horizontally.
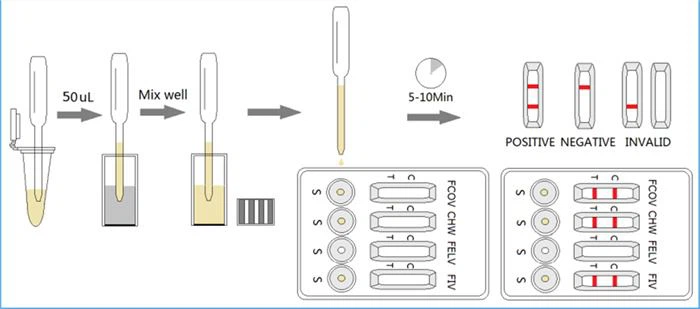
-Collect 50μL of the prepared specimen into a vial of FIV-FeLV-CHW assay buffer and mix well. Then drop 3 drops (approx. 120μL) of the diluted sample into each sample hole “S” of the test card, respectfully corresponding to the windows FIV, FeLV, CHW. Start the timer.
-Interpret the result in 5-10 minutes. Result after 10 minutes is considered as invalid.
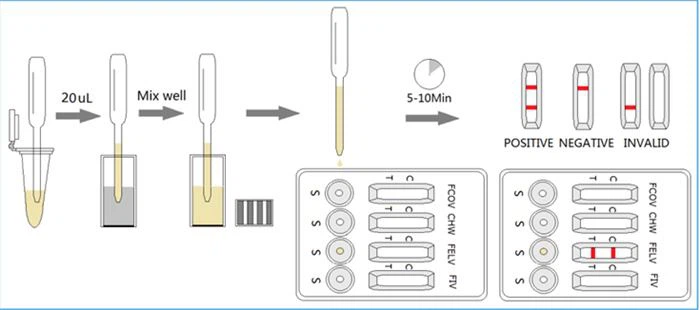
-Collect 20μL of the prepared specimen into a vial of FCoV Ab assay buffer and mix well. Then drop 3 drops (approx. 120μL) of the diluted sample into the sample hole “S” of the test card, corresponding to the windows FCoV. Start the timer.
-Interpret the result in 5-10 minutes. Result after 10 minutes is considered as invalid.
INTERPRETATION OF RESULTS
- Positive (+): The presence of both "C" line and zone "T" line, no matter T line is clear or vague.
- Negative (-): Only clear C line appear. No T line.
- Invalid: No colored line appears in C zone. No matter if T line appears.




